Clinical Unmet Need in Duchenne Muscular Dystrophy (DMD)
Treatment Recommendations Include Corticosteroids as Part of the Standard of Care in DMD1
- Corticosteroids slow the decline in muscle strength and function in DMD, in part due to effects on inflammation1,2
- Guidelines recommend initiating corticosteroids before substantial physical decline1
Chronic Corticosteroid Use Is Associated With Treatment-Limiting Adverse Effects3,4
Among ambulatory patients with DMD who have been treated with corticosteroids for >1 year4:
Adverse effects are the primary reason (in up to 65% of patients) for discontinuation of corticosteroid treatment5
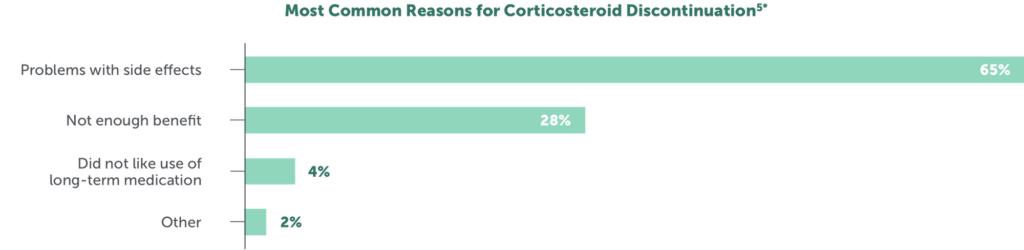
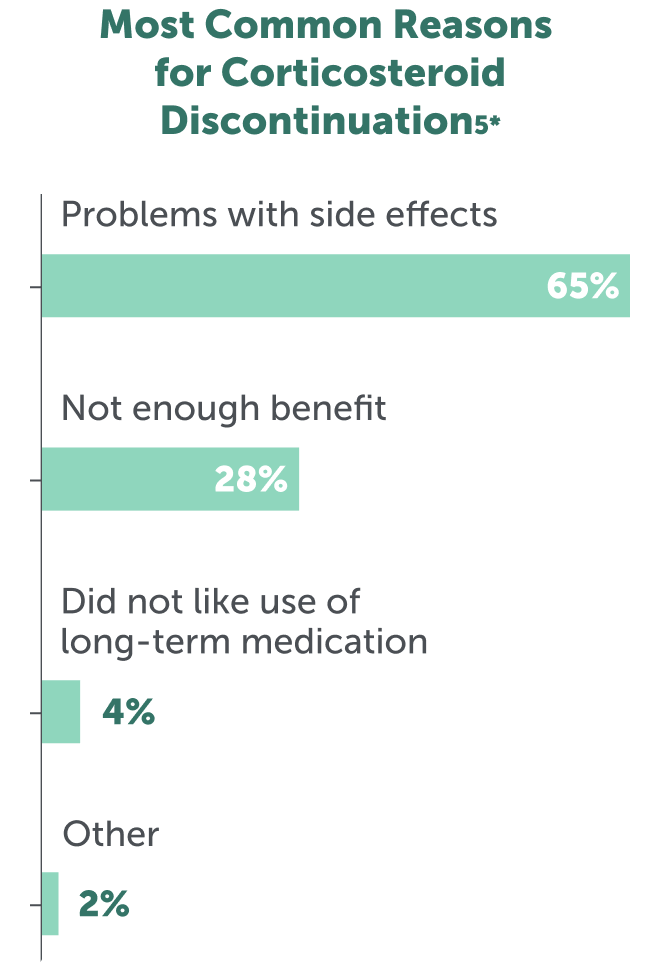
*Data from the Duchenne Registry including 1627 responses from individuals with DMD aged ≤35 years from the United States.5
Worry about side effects was the #1 reason (25%) for not initiating corticosteroid treatment5
Potentially Treatment-Limiting Adverse Effects*
- Changes in behavior, growth delay, and decline in bone health are important concerns related to chronic corticosteroid use1,4,6-8
- Additional treatment-limiting adverse effects may include cushingoid appearance, weight gain, hirsutism, and cataracts3-6
- Changes in behavior, growth delay, and decline in bone health are important concerns related to chronic corticosteroid use1,4,6-8
- Additional treatment-limiting adverse effects may include cushingoid appearance, weight gain, hirsutism, and cataracts3-6
Based on data from 277 patients (mean age, 10.9 years) treated with corticosteroids from the Cooperative International Neuromuscular Research Group Duchenne Natural History Study (CINRG-DNHS). Patients were considered treated with corticosteroids if they were taking corticosteroids for ≥1 year.4
Based on data from 97 patients with DMD aged 10 to <16 years from the Comprehensive Neuromuscular Center (CNC) at Cincinnati Children’s Hospital Medical Center treated with corticosteroids for ≥3 years.6
See why AGAMREE is different and why it might be the right choice for your patients
View clinical trial results for the efficacy and safety of AGAMREE in boys with DMD