Study Design & Efficacy
Study Design
AGAMREE® Study Design
The efficacy and safety of AGAMREE was studied in a multicenter, randomized, double-blind, placebo- and prednisone-controlled 24-week clinical trial1*

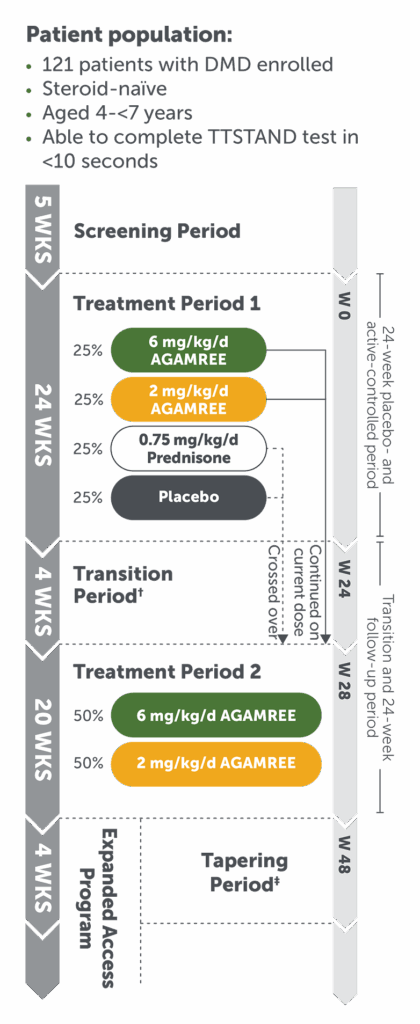
Study Design: Study 1 was a multicenter, randomized, double-blind, placebo- and active controlled clinical trial in 121 boys aged 4 to <7 years of age with a confirmed DMD diagnosis who were ambulatory and who had not been previously treated with corticosteroids.1 Patients were randomized to treatment with AGAMREE 6 mg/kg/d (n=30), AGAMREE 2 mg/kg/d (n=30), prednisone 0.75 mg/kg/d (n=31), or placebo (n=30) for 24 weeks.2 At baseline, patients had a mean age of 5.4 years, mean height of 109 cm, and mean weight of 20 kg.2
The uncontrolled study was conducted for an additional 24 weeks, making the total treatment time 48 weeks.2
For patients receiving prednisone or placebo; patients receiving AGAMREE remained on the same dose.1
For patients electing not to continue treatment past the end of the double-blind phase.1
Outcome Measures at Week 24
Primary Endpoint: TTSTAND velocity
Secondary Endpoints: 6MWT, TTRW velocity
Exploratory Endpoints: TTCLIMB velocity, NSAA
Clinical Safety Endpoints: Height percentile, BMI
Biomarker Safety Endpoints: Serum biomarkers of bone formation, adrenal suppression
6MWT, 6-Minute Walk Test; BMI, body mass index; DMD, Duchenne muscular dystrophy; NSAA, North Star Ambulatory Assessment; TTCLIMB, Time to Climb; TTRW, Time to Run/Walk; TTSTAND, Time to Stand.
Efficacy
AGAMREE Improved Muscle Strength and Motor Function
AGAMREE significantly improved motor function as assessed by the Time to Stand Test (TTSTAND velocity)1,2*
- Primary endpoint: AGAMREE 6 mg/kg/d improved TTSTAND velocity at Week 24 vs placebo (least-square mean difference vs placebo, 0.06 rises/s [P=0.002])2
- Prespecified secondary endpoint: AGAMREE 2 mg/kg/d improved TTSTAND velocity at Week 24 vs placebo (least-square mean difference vs placebo, 0.045 rises/s [P=0.017])2
- Improvements with AGAMREE 6 mg/kg/d and 2 mg/kg/d were clinically meaningful (MCID, >0.02 rises/s)1,3
TTSTAND velocity measures the time required to stand to an erect position from a supine position (floor).2
Baseline mean TTSTAND velocity, rises/s (SD): AGAMREE 6 mg/kg/d: 0.19 (0.06); AGAMREE 2 mg/kg/d, 0.18 (0.05); placebo, 0.20 (0.06).1
MCID, minimal clinically important difference; SD, standard deviation; SEM, standard error of the mean.
Prespecified Secondary Endpoints
AGAMREE significantly improved motor function as assessed by the 6-Minute Walk Test (6MWT)1,2‡
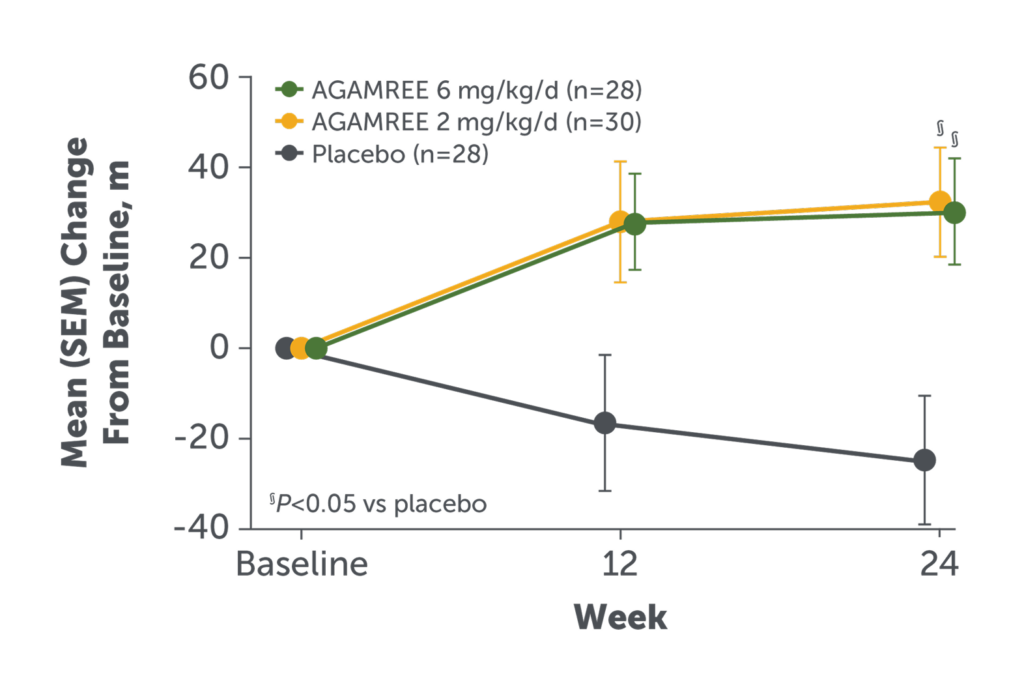
- AGAMREE 6 mg/kg/d and 2 mg/kg/d improved 6MWT vs placebo (least-square mean change from baseline vs placebo, 42 m [P=0.002] and 40 m [P=0.004], respectively)2
- Improvements with AGAMREE were clinically meaningful (MCID, >30 m)1
See study design for Study 1, above.
6MWT measures the distance a patient can walk on a flat hard surface in a period of 6 minutes.2
Baseline mean 6WMT, m (SD): AGAMREE 6 mg/kg/d: 313 (56); AGAMREE 2 mg/kg/d,
316 (58); placebo, 355 (78).1
AGAMREE significantly improved motor function as assessed by Time to Run/Walk 10 Meters (TTRW velocity)1,2¶
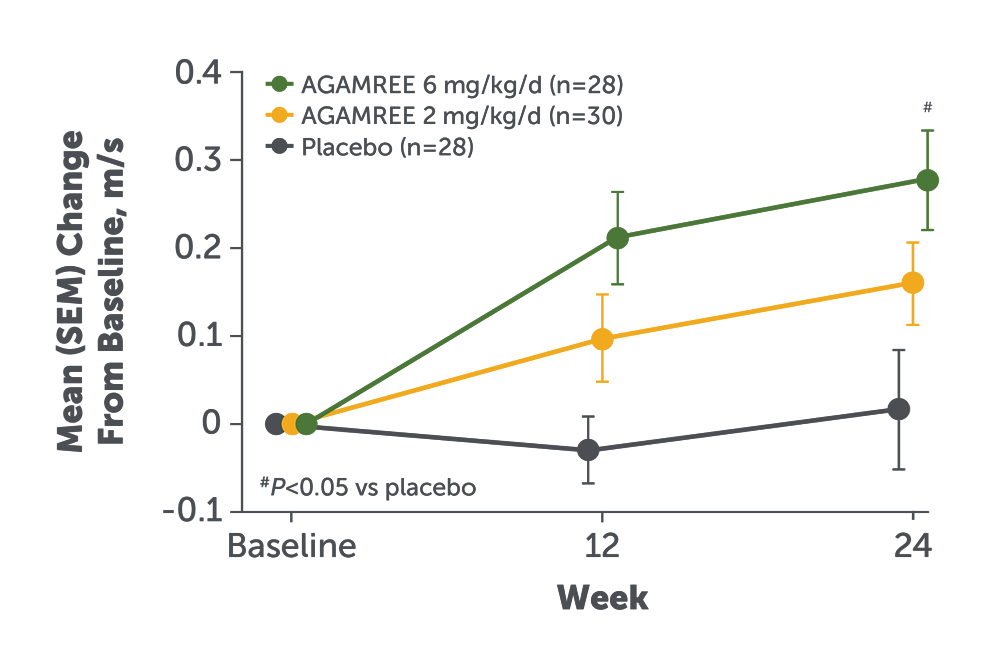
- AGAMREE 6 mg/kg/d improved TTRW velocity at Week 24 vs placebo (least-square mean change from baseline vs placebo, 0.24 m/s [P=0.002])2
- Improvement with AGAMREE was clinically meaningful (MCID, >0.21 m/s)3
See study design for Study 1, above.
TTRW velocity measures the time it takes a patient to run or walk 10 meters.2
Baseline mean TTRW velocity, m/s (SD): AGAMREE 6 mg/kg/d: 1.6 (0.4); AGAMREE 2 mg/kg/d, 1.6 (0.3); placebo, 1.7 (0.3).1
Exploratory Endpoints
AGAMREE improved motor function on NorthStar Ambulatory Assessment (NSAA)1*
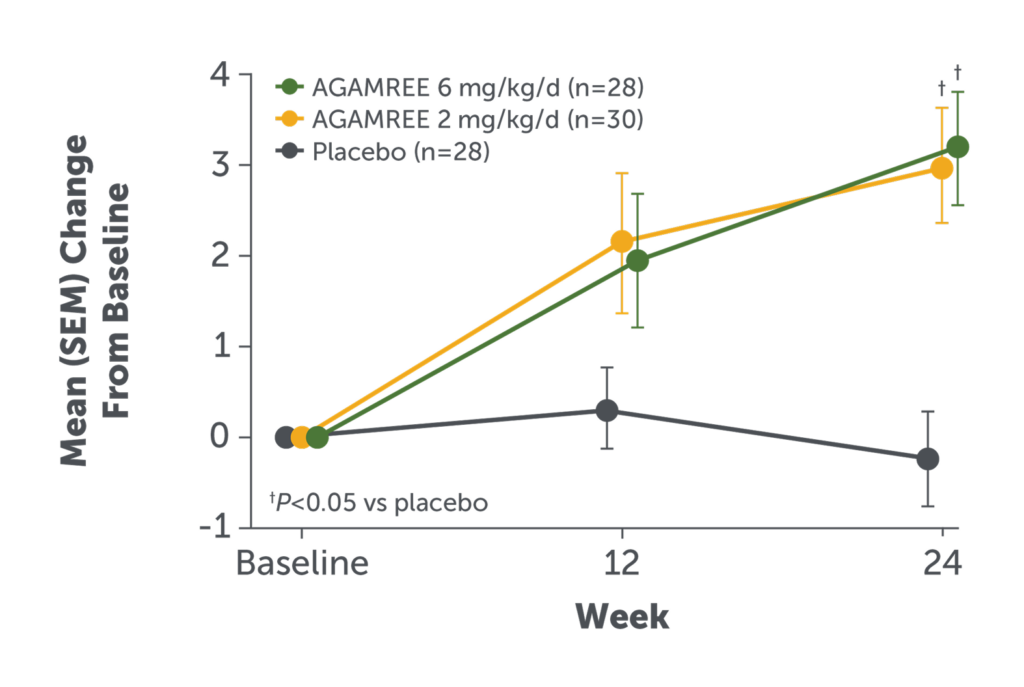
- AGAMREE 6 mg/kg/d improved NSAA total score at Week 24 vs placebo (least-square mean difference versus placebo 3.6)1
- Improvements in NSAA total score with AGAMREE 6 mg/kg/d were clinically meaningful (MCID, >2-3 points)4
The NSAA is a 17-item rating scale assessing motor function.5
Baseline mean NSAA score (SD): AGAMREE 6 mg/kg/d, 18.9 (4.1); AGAMREE 2 mg/kg/d, 17.2 (4.7); placebo, 18.9 (5.3).1
AGAMREE improved motor function as assessed by the Time to Climb Test (TTCLIMB velocity)1‡
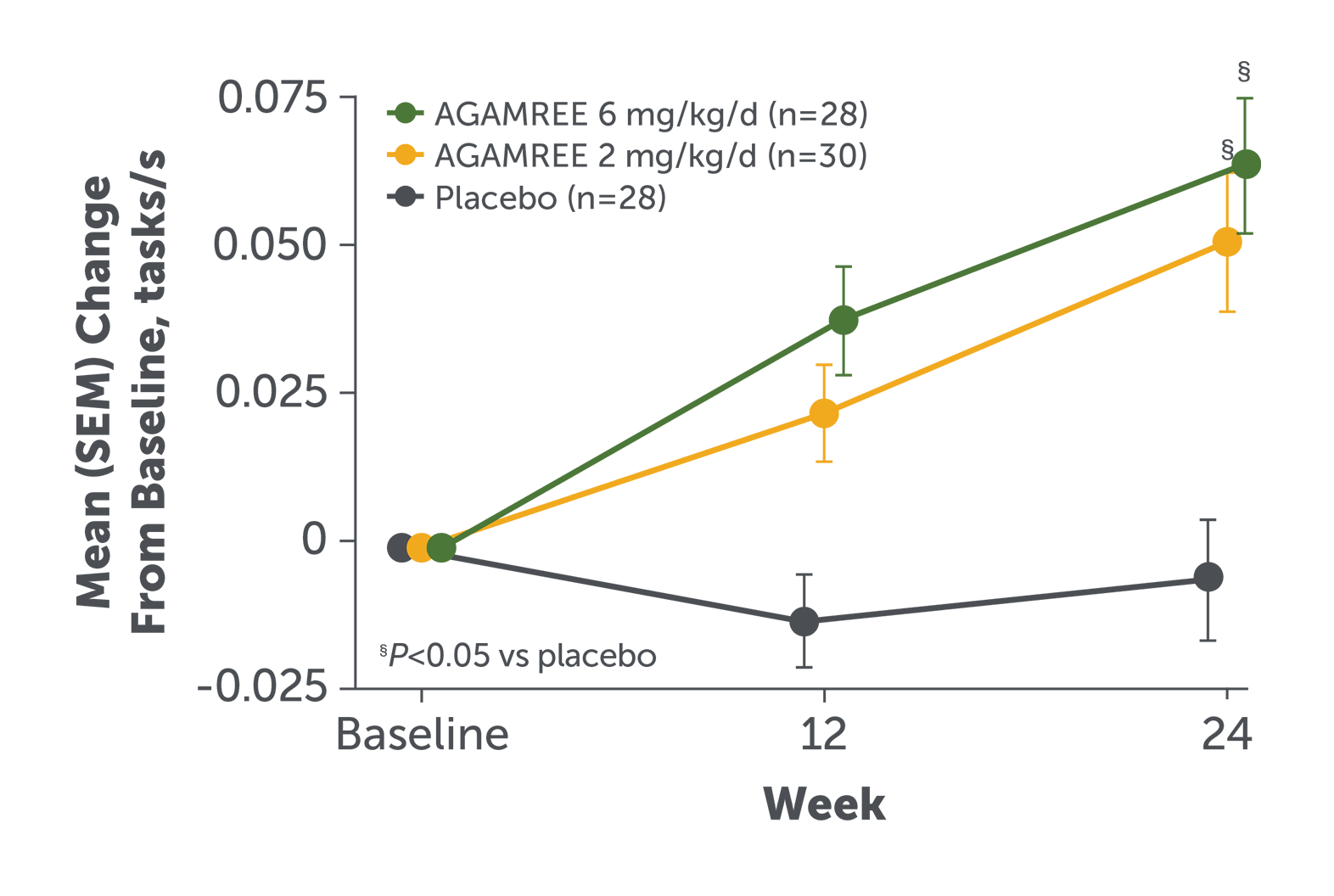
- AGAMREE 6 mg/kg/d improved TTCLIMB velocity at Week 24 vs placebo (least-square mean difference versus placebo 0.07 tasks/s)1
- Improvements in TTCLIMB velocity with AGAMREE 6 mg/kg/d were clinically meaningful (MCID, >0.035 tasks/s)3
TTCLIMB velocity measures the time to climb 4 stairs and assesses proximal (core) strength and motor function.1,6
Baseline mean TTCLIMB velocity, tasks/s (SD): AGAMREE 6 mg/kg/d, 0.21 (0.09); AGAMREE 2 mg/kg/d, 0.20 (0.05); placebo, 0.25 (0.09).1
Clinical Safety Endpoints
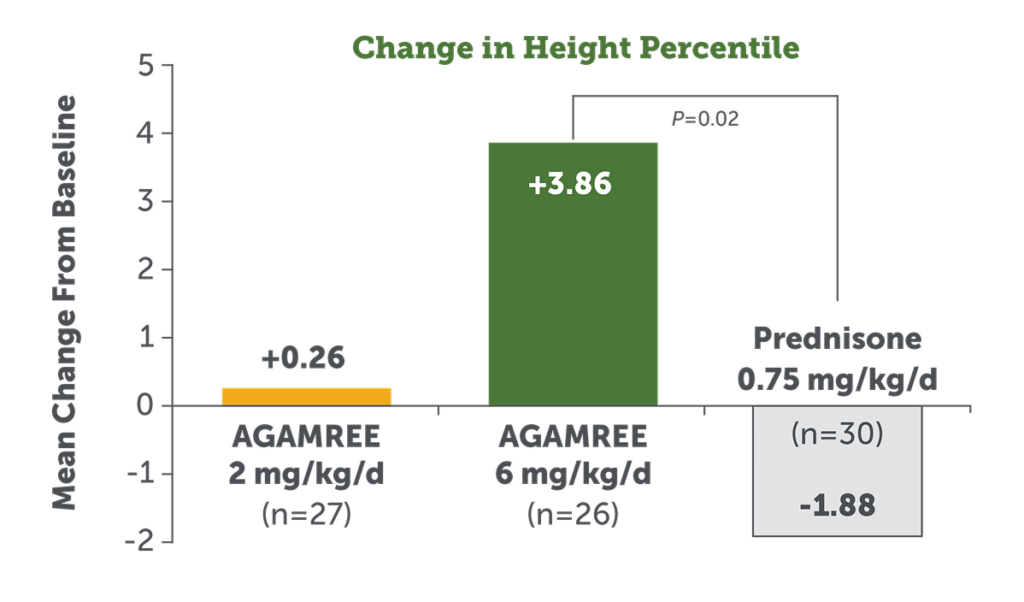
AGAMREE Positively Affected Height Percentile Compared to Prednisone Over 24 Weeks1
- AGAMREE 6 mg/kg/d significantly improved height percentile from baseline at Week 241
- Prednisone 0.75 mg/kg/d negatively affected height percentile1
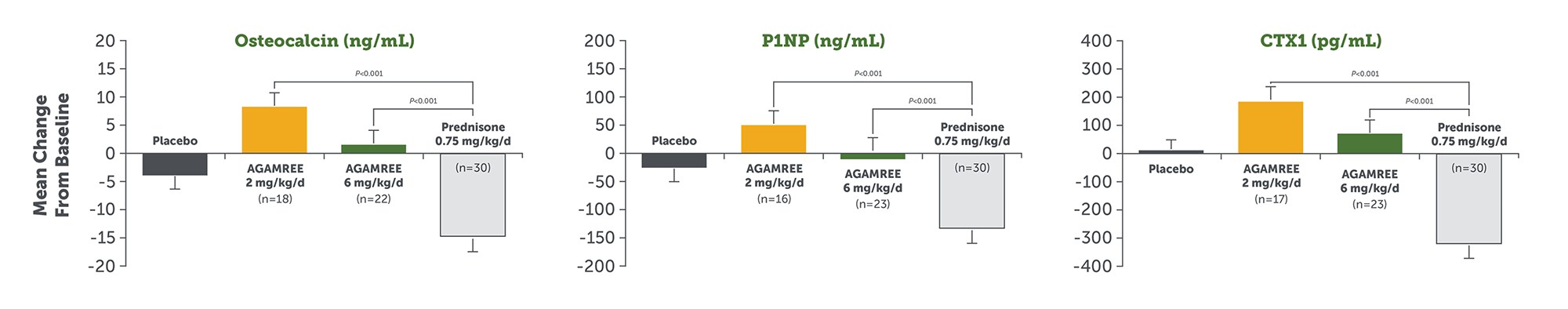
Biomarker Safety Endpoints
AGAMREE demonstrated no deleterious changes in bone biomarkers compared to prednisone over 24 weeks1,7*†
Additional data are needed to determine the long-term effects of AGAMREE on bone health.
Statistical analysis: MMRM of vamorolone groups vs prednisone group.
CTX1, C-terminal telopeptide of type 1 collagen; MMRM, mixed model of repeated measures; P1NP, procollagen type 1 intact N-terminal propeptide.
Figures adapted from: Hoffman E. PPMD Annual Conference 2021.
AGAMREE is an orange-flavored oral suspension dosed once daily
Enroll your patients in Catalyst Pathways® for access to a wide range of personalized support and programs