Long-Term Data & Safety
Long-Term Data
AGAMREE® 48-Week Follow-Up
The efficacy and safety of AGAMREE at 24 weeks were maintained over 48 weeks in boys with DMD8
- The clinically meaningful and statistically significant improvements in motor function on all 5 clinical outcomes at 24 weeks among patients taking AGAMREE 6 mg/kg/d were maintained at 48 weeks8
DMD, Duchenne muscular dystrophy; NSAA, North Star Ambulatory Assessment; TTCLIMB, time to climb 4 steps; TTRW, time to run/walk 10 m; TTSTAND, time to stand (from supine).
Clinical Safety Endpoints
Change in Height Over 48 Weeks
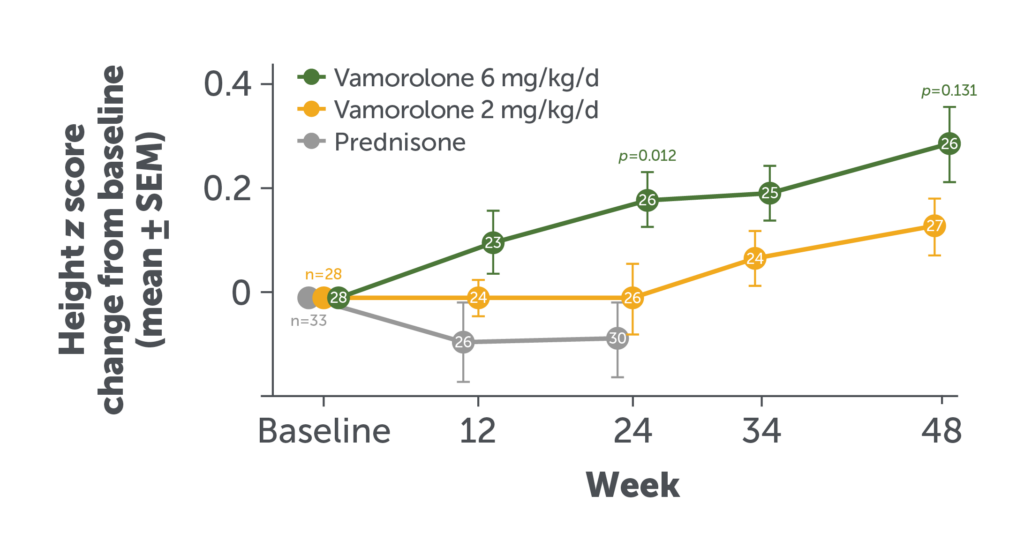
Among patients who received AGAMREE for 48 weeks, growth trajectories were normal with no significant dose-dependent differences.8
AESI, adverse event of special interest; BMI, body mass index; SEM, standard error of the mean.
Change in BMI Over 48 Weeks
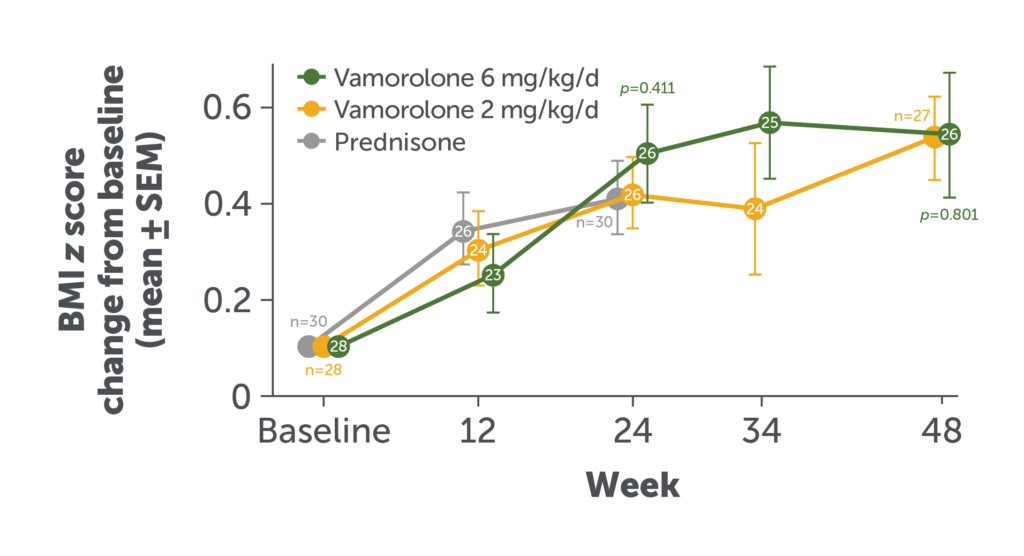
Among patients who received AGAMREE for 48 weeks, BMI increased through Week 24 but stabilized through Week 48.8
Bone Biomarkers
Among patients who received prednisone through Week 24 and AGAMREE 6 mg/kg/d or AGAMREE 2 mg/kg/d through Week 48, the decrease in serum bone turnover markers (osteocalcin, P1NP, and CTX1) was quickly reversed after tapering prednisone and switching to AGAMREE8
CTX1, C-terminal telopeptide of type of type 1 collagen; P1NP, procollagen type 1 intact N-terminal propeptide.
Multicenter, Nonrandomized, Controlled, 24-Month, Open-Label Extension Trial
AGAMREE efficacy was assessed with historical controls on traditional corticosteroids9
Participants
- Boys aged 4.5 to 7.5 years with DMD not previously treated with corticosteroids who completed a 6-month, open-label dose-finding study for vamorolone were enrolled in the 24-month, open-label extension9
30-Month, Open-Label Treatment
- In the 6-month dose-finding study, patients were assigned to receive vamorolone at 1 of 4 doses9:
0.25 mg/kg/d
0.75 mg/kg/d
2 mg/kg/d
6 mg/kg/d
- Dose escalations up to 6 mg/kg/d were permitted during the 24-month, long-term extension; decreases were also allowed based on tolerability9
- Throughout the studies, vamorolone was provided as a 4% flavored oral suspension taken daily at breakfast with a 240 mL glass of whole milk, or an equivalent high-fat food portion9
Population
Vamorolone group9
46 corticosteroid-naive boys with DMD (aged 4.5 to 7.5 years)
Mean age, 5.3 years
Control groups9
Boys with DMD (aged 4.5 to 7.5 years) treated with corticosteroids for 6 months prior to the comparison baseline visit from:
- Cooperative International Neuromuscular Research Group (CINRG) Duchenne Natural History Study (DNHS; n=75)
Prespecified analyses focused on patients in the higher-dose group, which included those patients initially assigned to, and maintained on, a dose of 2 mg/kg/d to 6 mg/kg/d for up to 30 months.9
Lower-dose group9*
11 initially assigned to 0.25 mg/kg/d
12 initially assigned to 0.75 mg/kg/d
*Escalations permitted up to 6 mg/kg/d.
Higher-dose group9†
12 initially assigned 2 mg/kg/d
11 initially assigned 6 mg/kg/d
Baseline characteristics (mean)
Age: 5.83 y | Weight: 21.98 kg | Height: 111.8 cm | BMI: 17.68
Maintained on >2 mg/kg/d throughout the 30-month treatment period, with escalations permitted up to 6 mg/kg/d.
Results
Overview9
At the end of the 30-month trial period:
11 patients treated at 2.0 mg/kg/day
3 patients treated at 4.0 mg/kg/day
27 patients treated at 6.0 mg/kg/day
In this 30-month, open-label extension trial using historical controls, efficacy remained comparable to traditional corticosteroids. Vamorolone was well tolerated over the 30-month period. Patients who received vamorolone experienced fewer adverse outcomes compared with the DNHS control group.9
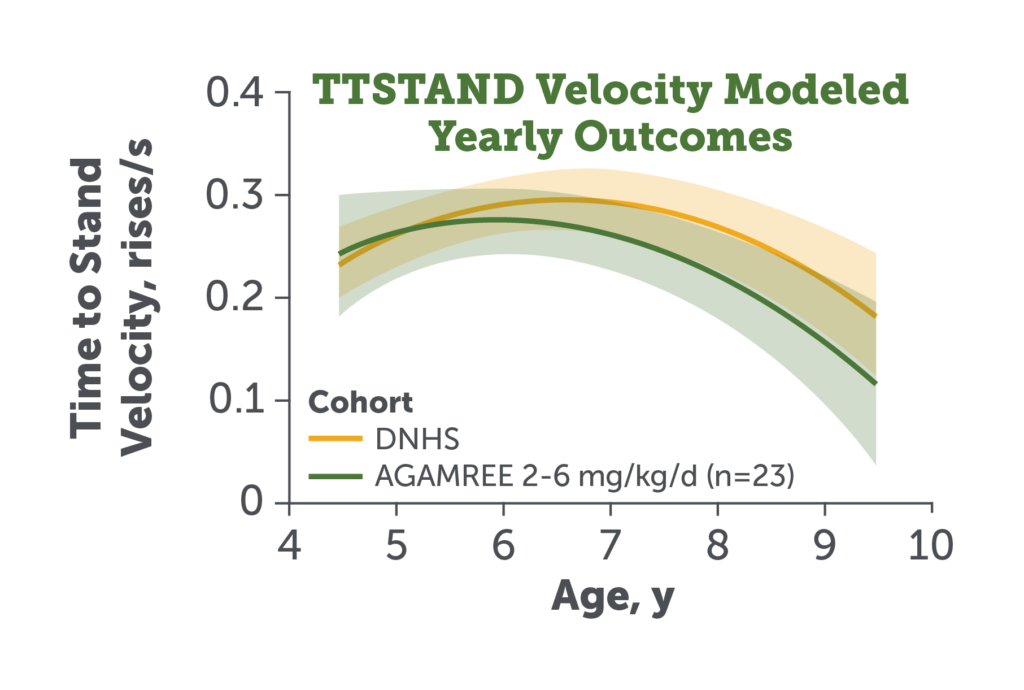
- The study primary endpoint TTSTAND velocity remained comparable in patients treated with AGAMREE 2-6 mg/kg/d (LTE)* vs DNHS control patients† receiving traditional corticosteroids9
AGAMREE-treated cohort: Corticosteroid-naive patients with DMD aged 4.5 to 7.5 years (n=46).
Mean age: 5.8 years; mean weight: 22.0 kg; mean height: 111.8 cm; mean BMI: 17.9
Patients with DMD treated with corticosteroids for 6 months prior to comparison baseline visit aged 4.5 to 7.5 years (n=75).9
Figure adapted from Mah JK et al. JAMA Netw Open. 2022;5(1):e2144178.
LTE, long-term extension.
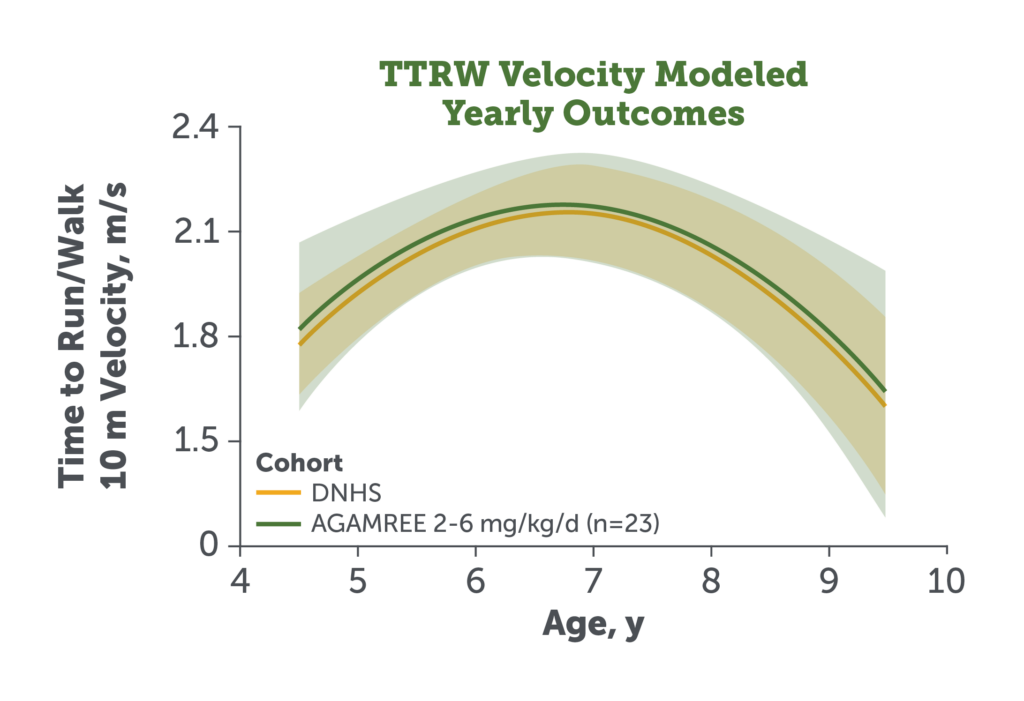
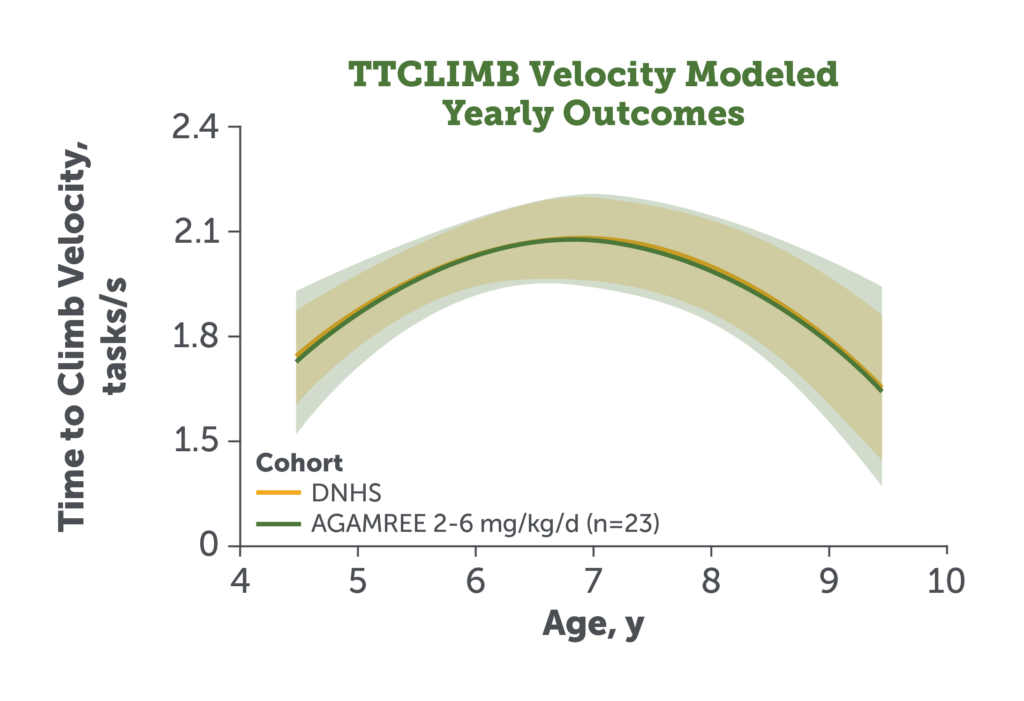
- The study secondary endpoints TTRW and TTCLIMB velocity remained comparable in patients treated with AGAMREE 2-6 mg/kg/d (LTE) vs DNHS control patients receiving traditional corticosteroids9
Figures adapted from Mah JK et al. JAMA Netw Open. 2022;5(1):e2144178.
Safety
AGAMREE Was Well Tolerated in Clinical Studies
- The most common adverse reactions (>10% and greater than placebo) in boys with DMD treated with AGAMREE in Study 1 were cushingoid features, psychiatric disorders, vomiting, weight increased, and vitamin D deficiency2
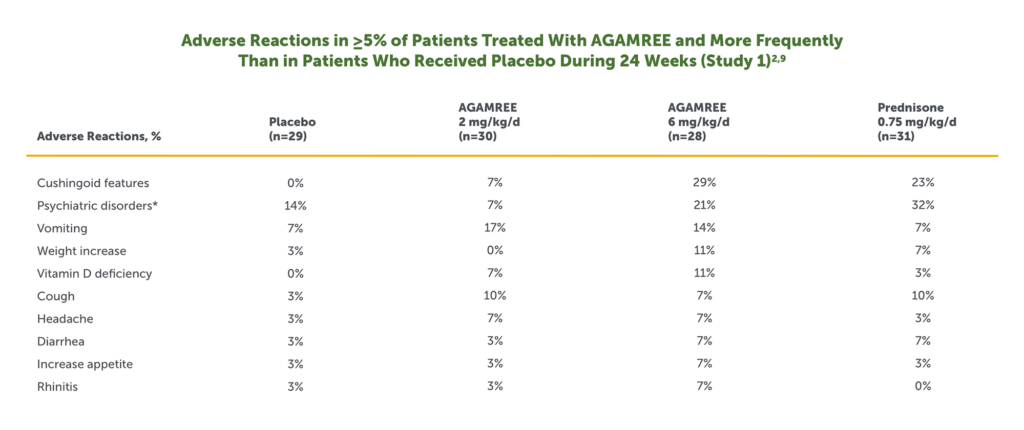

*Includes the following adverse reactions that occurred more frequently in the AGAMREE group than in placebo: abnormal behavior, aggression, agitation, anxiety, irritability, mood altered, sleep disorder, and stereotypy.
- In a separate open-label safety study of pediatric patients with DMD aged 2 to <4 years (n=16) and pediatric patients aged 7 to <18 years (n=16), adverse reactions were similar to Study 12
The safety profile of AGAMREE at 48 weeks was consistent with its profile at 24 weeks8
- Fewer patients reported AESIs in Period 2 compared with Period 1, with the largest decrease seen in behavior problems8
AGAMREE is an orange-flavored oral suspension dosed once daily
Enroll your patients in Catalyst Pathways® for access to a wide range of personalized support and programs




